Omnigen®
Information for clinicians

What is Omnigen?
Omnigen is a dry and stable human amniotic membrane-derived material. Amniotic membrane can be used to aid the natural healing process in ophthalmic conditions and soft tissue-damage situations.
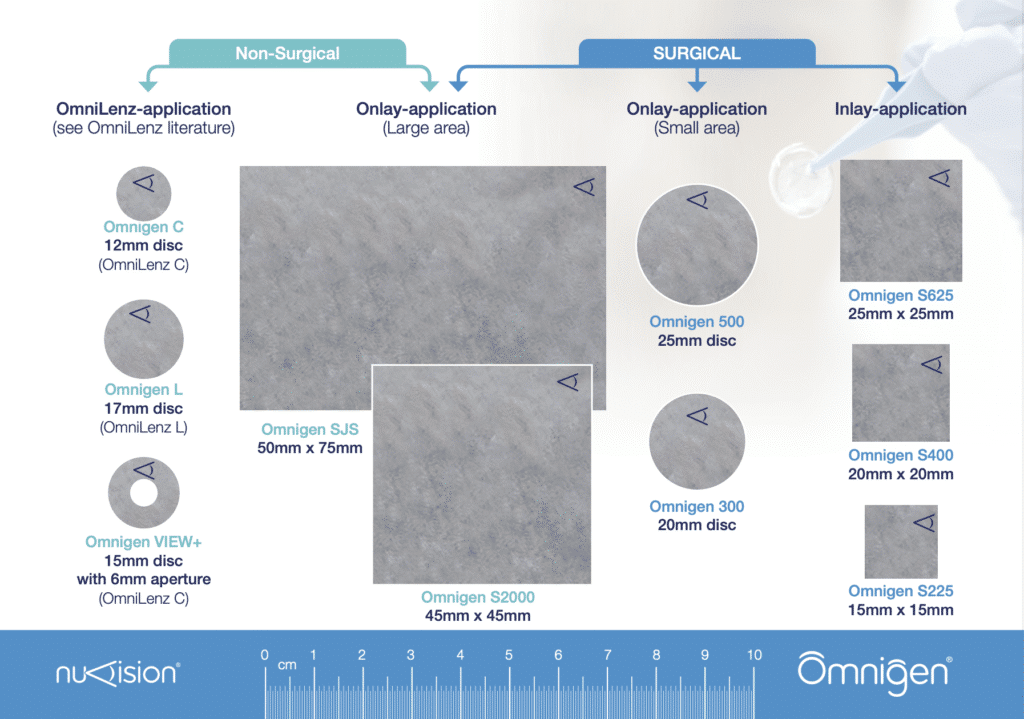
- Supplied in a convenient range of shapes and sizes
- Shipped and stored between 2ºC to 25ºC
- Applied to the wound dry for in-vivo rehydration
- Ready for use at point-of-care
- Accessible in an outpatient setting by ambulatory application
NuVision use a patented and unique process called Tereo to produce Omnigen which ensures the barrier function benefits of amniotic membrane are retained to facilitate the natural healing process.
Omnigen can be applied in a suture free or ambulatory setting, or surgically as a:
- Permanent inlay (graft): supporting the structure and re-growth of natural tissue; or
- Temporary onlay (patch): acting as a biological dressing to protect the wound, physically prevent inflammation development and progression, prevent adhesion development, and support pain management, whilst facilitate tissue recovery

Omnigen is approved for treatment on the National Health Service (NHS) and at certain private clinics. Your healthcare professional will have information on how to access Omnigen.

Omnigen application allows for the natural benefits of amniotic membrane to be delivered to the ocular surface.
Key benefits of amniotic membrane’s barrier function allow its transplantation to:
- Help reduce inflammation development and progression
- Provide a protective and supportive environment to facilitate natural healing and recovery
- Physically alleviates pain by protecting the ocular surface
- Physically prevents the development of adhesions
OmniLenz application of Omnigen can be used to manage conditions that affect the ocular surface. For more severe situations that require mucosal surface coverage, larger pieces of Omnigen can be applied in a suture free setting.
Together, these strategies provide total ocular surface coverage options that can be considered as part of early acute and prophylactic treatment strategies.
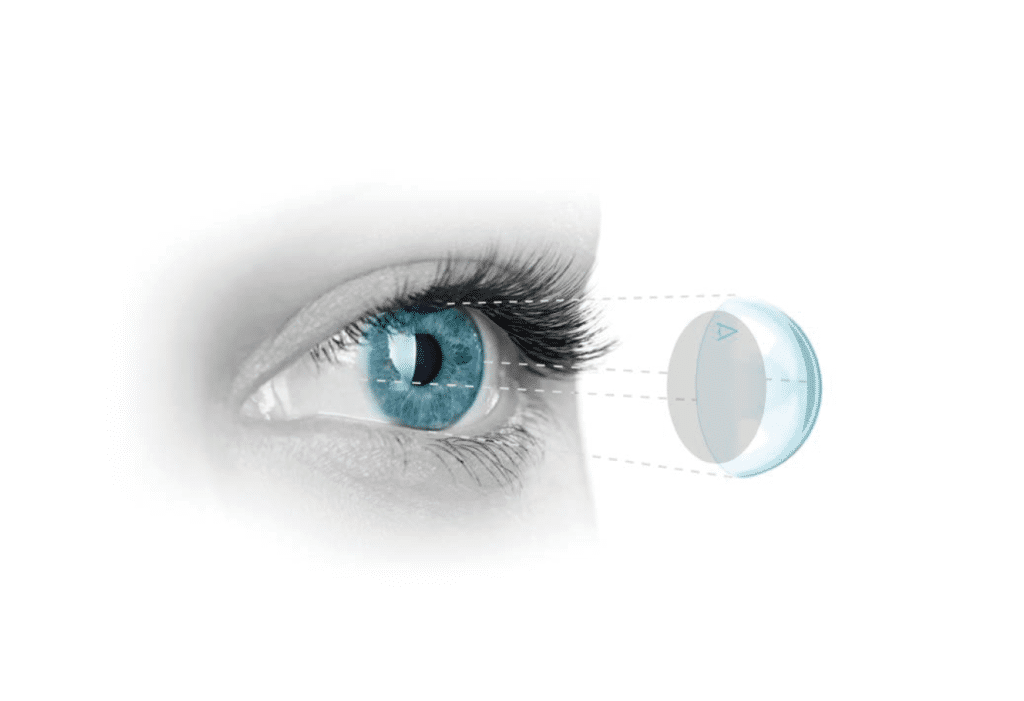
Applications of Omnigen
Omnigen’s barrier function can be used as an onlay (patch) or inlay (graft).
- Patch applications are applied epithelial side down as a temporary biological dressing that protects and nurtures the wound, providing a physical barrier to inflammation, adhesions and pain, and to aid in epithelial regrowth under the membrane.
- Graft applications are applied epithelial side up to provide a natural substrate for cellular re-growth and a scaffold for tectonic support of damaged tissue. A graft is permanent and integrates over time.




The science
The amniotic membrane is the inner, foetal-derived, avascular layer of the foetal membrane. It surrounds, protects, supplements and nurtures the foetus during pregnancy. The amniotic membrane is responsible for normal growth and development of the foetus.
During pregnancy, the amniotic membrane acts as a physical barrier between the mother and foetus, preventing the maternal immune responses and inflammatory cell infiltration that could harm the baby. This immuno-regulatory (immune privilege) property of amniotic membrane is believed to also protect against tissue rejection and inflammation and nurture the healing process when amniotic membrane is used as a tissue transplant.
The barrier function of amniotic membrane is associated with beneficial properties that support natural healing and prevent the infiltration of damaging immune cells into open wounds.
When amniotic membrane is delicately processed and preserved using techniques such as the Tereo process the important barrier function and membrane architecture can be preserved.
How to use Omnigen
Omnigen is pliable and easy to manipulate and cut, if necessary in its dry format
It is supplied in bespoke packaging and can be applied directly to the wound where it rehydrates from ambient and ocular moisture. The epithelial side is marked for confident application.
Omnigen comes in a range of convenient pre-cut sizes for ease of use and reduced waste.
Where appropriate, Omnigen can be applied in the outpatient setting using our optimised bandage contact lens OmniLenz.

How to store Omnigen
Omnigen should be stored between 2ºC to 25ºC and out of direct sunlight.
If you are based in the United Kingdom, please contact the NuVision team to discuss if you are able to store Omnigen at your establishment.