OmniLenz® bandage contact lens
Information for clinicians

What is OmniLenz?
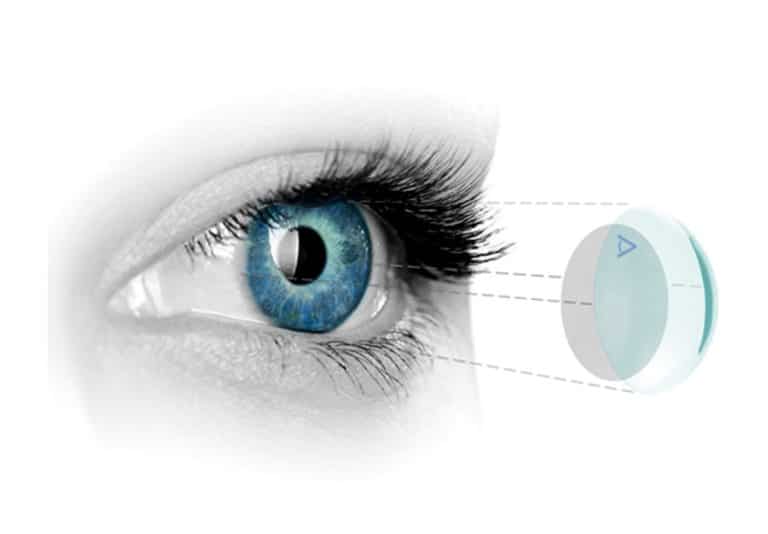
OmniLenz® is a bandage contact lens (BCL) that allows suture-free transplantation (onlay-barrier application) of Omnigen to the ocular surface in an ambulatory and outpatient setting.
Suture-free transplantation of Omnigen means patients benefit from the barrier function of Omnigen without the need for surgery. Suture-free transplantation of Omnigen is suitable for a range of applications.
- Simple 4-6 minute procedure
- Suture-free technique
- Accessible for a range of aetiologies
- Available in non-elective emergency and outpatient settings
- Earlier intervention for better outcomes
- Available in two sizes for a wider range of treatment options

How it works
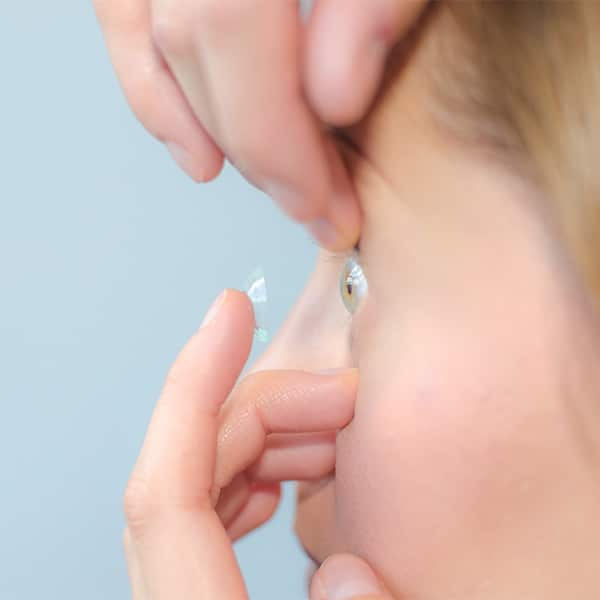
OmniLenz® is applied to the surface of the eye in a similar manner to a standard BCL. This means Omnigen can be applied routinely in an outpatient setting where surgery is not available. OmniLenz® application of Omnigen replaces the need for sutures, preventing iatrogenic damage to the compromised ocular surface.
OmniLenz® is derived from a high-water content soft material bandage contact lens base which sits securely on the ocular surface.
The simplicity of application means non-surgical healthcare professionals can be trained to apply OmniLenz® giving patients immediate access to care. OmniLenz should be used in line with the instructions for use.
Outpatient application of Omnigen means more than convenience, it allows unscheduled and emergency suture free intervention to facilitate:
- Management of ocular damage that could cause visual impairment
- Success in the management of refractory conditions
- Efficient and effective care pathway
- More patients have access to treatment
- Reduced healthcare resource utilisation and cost
Video
How to apply Omnigen using OmniLenz

Indications for use
OmniLenz® (Onlay-barrier amniotic membrane transplantation) can be considered in a range of settings.
OmniLenz may not be suitable for use in patients who exhibit abnormal blinking, excessive eye rubbing or who are unable to give informed consent.

Why OmniLenz?
OmniLenz® allows for the simple application of Omnigen to the surface of the eye in an ambulatory setting. This saves time, resources and cost while ensuring intervention is received as quickly as possible.
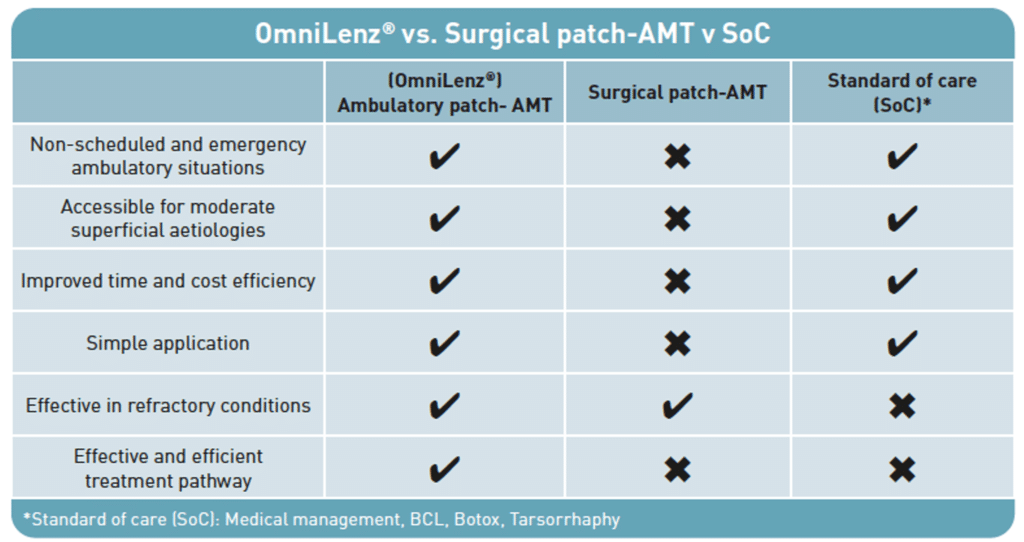
Outpatient transplantation of Omnigen has a range of benefits when compared to use of amniotic membrane in a surgical setting and standard of care.
Compliance with Human Tissue Authority (HTA) regulations
Ambulatory transplantation of Omnigen® using OmniLenz® is considered a human tissue transplantation procedure, therefore use of Omnigen® and OmniLenz® should comply with traceability requirements within your territory.
OmniLenz® is a CE marked product and should be used in line with the instructions for use.
Activity recording involving the application of Omnigen with OmniLenz in the United Kingdom will be based on two NHS OPCS codes: C46.6: Amniotic membrane graft to cornea and C51.5: Placement of therapeutic contact lens on to cornea.