Attendees at this week’s prestigious Saudi Ophthalmology annual meeting - So2023 (link) from 19-21st October in Riyadh will learn about the latest clinical research outcomes on the sutureless, in-office application of Omnigen amniotic membrane as a new treatment for moderate-to-severe dry eye disease (DED).
Managing DED symptoms is challenging. One in four of us will experience this debilitating condition with symptoms including sore, gritty, itchy, watery, or irritated eyes, eye fatigue/strain, and potential discharge.
Professor Wolffsohn at the College of Health & Life Sciences, Optometry & Vision Science Research Group (OVSRG), Aston University, a renowned expert in DED and contact lenses, recently concluded a world-first randomized controlled clinical trial for treating moderate-to-severe DED binocularly. This trial, in collaboration with Dr. Sònia Travé-Huarte, employed the amniotic membrane (Omnigen-VIEW) applied using a specialized bandage contact lens (OmniLenz-VIEW).
Dr. Travé-Huarte will present these findings in person on Thursday, 19th October, during the Anterior Segment (The Spectrum of Ocular Surface Disease) session. The “Dry eye OmniLenz application of Omnigen Research Study (DOORS)” results indicate that a 1-week bilateral treatment with Omnigen-VIEW leads to rapid, significant, and sustained improvement in patient symptoms and ocular surface health, including nerve regeneration. The research team considers Omnigen-VIEW a promising new treatment for individuals with moderate-to-severe DED.
NuVision’s International Business Development Manager (IBDM) will also be present, supporting their regional partner, Aksia Health (link) showcasing the Omnigen products that allow innovative surgery free outpatient treatment.
In collaboration, Aston University is recruiting for the next phase of clinical evaluation, aiming to establish Omnigen as a routine treatment for DED. Read more.
About NuVision Biotherapies
Located in MediCity, Nottingham, NuVision Biotherapies is a regenerative medicine company originating from the University of Nottingham. Founded in 2015 with investment from Mercia Fund Management, NuVision aims to develop and introduce pioneering regenerative therapies.
About Omnigen
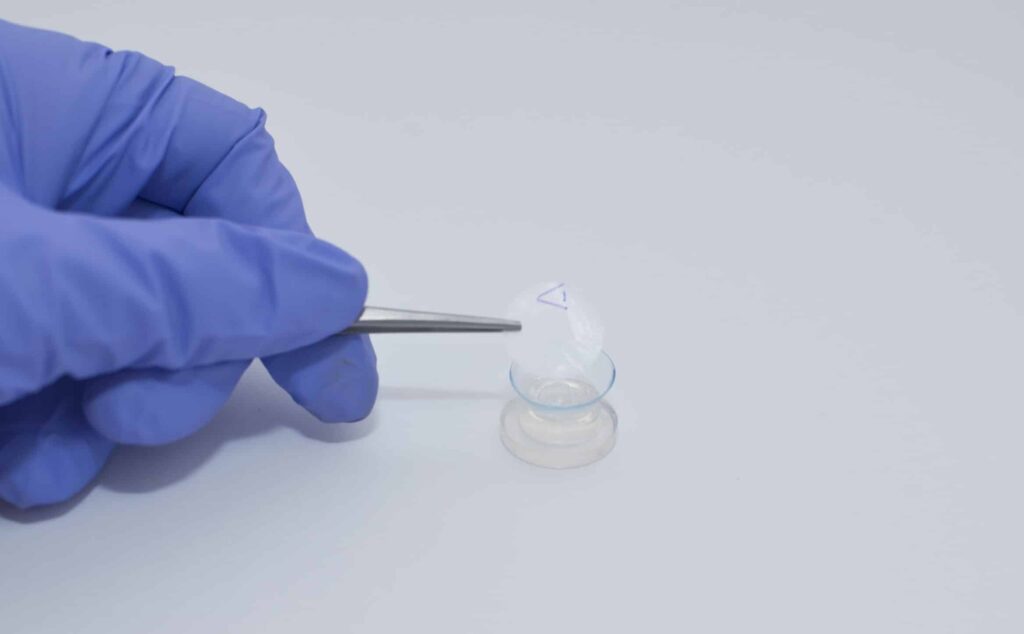
Omnigen, a patented dry preparation of the human amniotic membrane, originates from the innermost layer of the placental sac protecting the foetus during pregnancy. Developed at the University of Nottingham’s Academic Ophthalmology department, NuVision’s Tereo manufacturing process transforms this birth by-product into a sterile, stable dry therapy. Easy to ship worldwide, Omnigen quickly rehydrates upon application, promoting tissue repair in numerous ways. It serves as a unique, long-storage, “off-the-shelf” healing product, especially benefiting patients with ocular surface disease and other unmet medical needs. The Omnigen VIEW range includes central aperture windows, allowing the product’s application without obscuring vision.
About OmniLenz
Manufactured exclusively for NuVision by Menicon, UK, OmniLenz is a custom bandage contact lens created to hold Omnigen on the ocular surface without the need for sutures or surgery. In just a 4–6-minute outpatient procedure, healthcare professionals can harness the healing power of the amniotic membrane, offering a new treatment avenue for various ocular surface diseases.
About Aksia Health
Based in the UAE, Aksia Health Care FZC is a regional healthcare firm. Since 2016, it has been actively operating in the Middle East and North Africa (MENA) region and the Gulf Cooperation Council (GCC) as their primary area, specializing in marketing, sales, and the distribution of diverse speciality therapeutics, including rare diseases, plasma-derived products, and ophthalmological surgical solutions.





